Blood Buffer Biology Definition
A buffer system consists of a weak acid the proton donor and its conjugate base the proton acceptor. An example of a buffer solution is bicarbonate in blood which maintains the bodys internal pH.
 Buffers Definition Overview Expii
Buffers Definition Overview Expii
Therefore we want an expression for the.
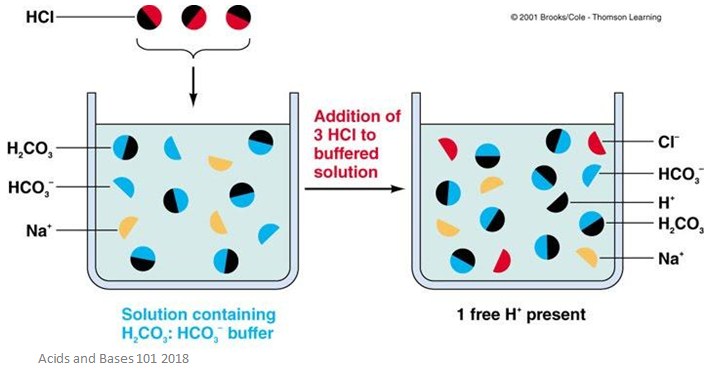
Blood buffer biology definition. Learn more about the components and function of blood. The Carbonic-Acid-Bicarbonate Buffer in the Blood By far the most important buffer for maintaining acid-base balance in the blood is the carbonic acid-bicarbonate buffer. A buffer consists of a weak acid and its conjugate base or a weak base and its conjugate acid.
For the body to function properly it is essential that there is tight pH regulation which maintains the body generally at a neutral pH of 74. The simultaneous equilibrium reactions of interest are. The dissolved carbon dioxide and bicarbonate ion are at equilibrium Eq.
Can be blood vessels to the leg handlungs or even brain. Blood contains specialized cells that serve particular functions. The stronger the acid the more readily it will dissociate into protons and anions in water where most biological reactions take place.
They are used for neutralizing acidic aqueous solutions. Blood that is too acidic or basic can damage bodily tissues. When CO 2 enters the venous blood the small decrease in pH shifts the ratio of acid to salt in all the buffer pairs.
The Carbonic-Acid-Bicarbonate Buffer in the Blood. Buffer Definition - Chemistry and Biology DEFINITION A buffer is an aqueous solution consisting of a mixture of a weak acid its salt or a weak base its salt that resist a change in pH on the addition of either acid or base. It is the main buffer in blood plasma and consists of bicarbonate HCO 3 and carbonic acid H 2 CO 3.
The most well known anticoagulants are Warfarin and Low Molecular Heparin which is given in a shot not per os like WArfarin. Anticoagulants given in order to avoid blood clots in your systems. These cells are suspended in a liquid matrix known as plasma.
A buffer system is a solution that resists a change in pH when acids or bases are added to it. Cells and organisms maintain a specific and constant cytosolic pH keeping biomolecules in their optimal ionic state usually near pH 7. Buffer solutions help in the adjustment of the nature of blood.
The blood buffers consists of the plasma proteins hemoglobin oxy-hemoglobin bicarbonates and inorganic phosphates. Our blood is a buffer system that keeps pH between 735 and 745. 49 votes See 1 more reply.
Blood as a Buffer Solution. The bicarbonate buffer neutralizes stronger dietary and metabolic acids HA converting them into weak bases A with the increase in H 2 CO 3. The bicarbonate buffer system in the blood maintains a balance between bicarbonate and carbon dioxide ions and deterimnes the pH of the blood.
Note- A lot of biological chemical reactions need a constant pH for the reaction to proceed. Blood itself tends to be a buffer solution by keeping its pH value constant. It is important that the pH does not stray too far from this range.
When the ratio is shifted to form more of the acid cations become available to form additional bicarbonates. Bufer a substance that by its presence in solution increases the amount of acid or alkali necessary to produce a unit change in pH. Most buffers consist of a weak acid and a weak base.
A buffer is rather simple by definition. In this way a biological buffer helps maintain the body at the correct pH so that biochemical processes continue to run optimally. A biological buffer is an organic substance that has a neutralizing effect on hydrogen ions.
Its a solution that contains an acid matched with an equal-strength or conjugate base. The bicarbonate buffer system is an acid-base homeostatic mechanism involving the balance of carbonic acid H 2 CO 3 bicarbonate ion HCO 3 and carbon dioxide CO 2 in order to maintain pH in the blood and duodenum among other tissues to support proper metabolic function. Human blood contains a buffer of carbonic acid H 2 CO 3 and bicarbonate anion HCO 3- in order to maintain blood pH between 735 and 745 as a value higher than 78 or lower than 68 can lead to death.
Blood fluid that transports oxygen and nutrients to cells and carries away carbon dioxide and other waste products. Alkaline or basic buffer solutions are those that have strong alkalis and weak acids in the mixture. Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes.
In this buffer hydronium and bicarbonate anion are in equilibrium with carbonic acid. In practice a buffer solution contains either a weak acid and its conjugate base or a weak base and its conjugate acid. Furthermore the carbonic acid in the first equilibrium can decompose into CO 2 gas and water resulting in a second equilibrium system between carbonic acid and water.
We are interested in the change in the pH of the blood. Remember that acids donate protons and bases accept protons. Some heard conditions for example like damaged valves can cause blood to clot on them and then theres a big risk that itll detach itself and move around your body until itll get stuck and block the blood vessel.
By far the most important buffer for maintaining acid-base balance in the blood is the carbonic-acid-bicarbonate buffer.
 Buffer Solutions Biochemistry The Biology Notes
Buffer Solutions Biochemistry The Biology Notes
 Pin On Montessori Eii Chemistry
Pin On Montessori Eii Chemistry
 Pin By Draw It To Know It Medical On Https Drawittoknowit Com Medical School Studying Science Notes Cell Biology
Pin By Draw It To Know It Medical On Https Drawittoknowit Com Medical School Studying Science Notes Cell Biology
 Types Of Hydrogen Isotopes Chemistry Basics Science Chemistry Chemistry
Types Of Hydrogen Isotopes Chemistry Basics Science Chemistry Chemistry
 Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions
Buffer Solution Preparation Of Buffer Solution Acidic Basic Buffer Buffer Action Buffer Solution Electron Configuration Solutions
 Polysaccharides Structure Examples Notes Easy Biology Class Biology Class Biochemistry Biology
Polysaccharides Structure Examples Notes Easy Biology Class Biology Class Biochemistry Biology
 Digital Kemistry Best Chemistry Animated Blogs What Is Valency In Chemistry Definition Example 11th Chemistry Chemistry Biochemistry
Digital Kemistry Best Chemistry Animated Blogs What Is Valency In Chemistry Definition Example 11th Chemistry Chemistry Biochemistry
 Acid Base Balance Anatomy And Physiology Ii
Acid Base Balance Anatomy And Physiology Ii
 Full Size Picture Fig107b Jpg Medical Dictionary Cell Membrane Medical
Full Size Picture Fig107b Jpg Medical Dictionary Cell Membrane Medical
 Acid Base Buffers Facts Summary Definition Chemistry Revision
Acid Base Buffers Facts Summary Definition Chemistry Revision
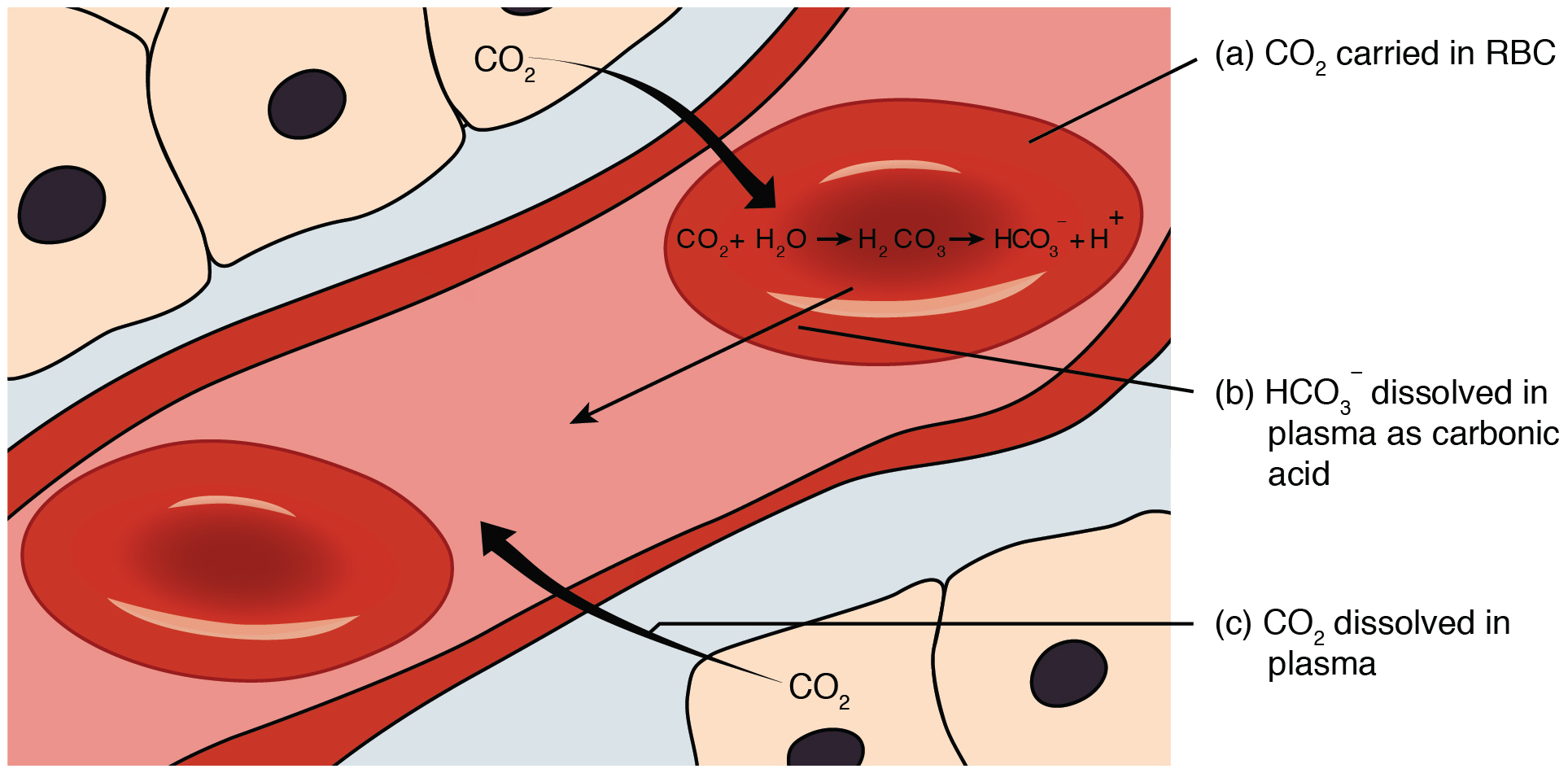 Bicarbonate Buffer System Wikipedia
Bicarbonate Buffer System Wikipedia
 Bicarbonate Buffer System Example Of Multiple Equilibria Teaching Chemistry Medical School Studying Biochemistry
Bicarbonate Buffer System Example Of Multiple Equilibria Teaching Chemistry Medical School Studying Biochemistry
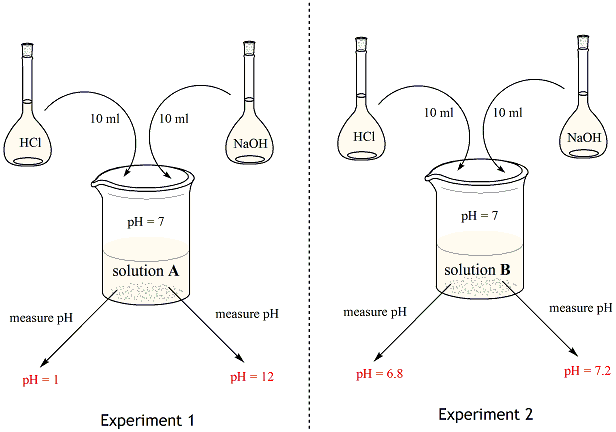 Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
Chemistry Of Buffers And Buffers In Our Blood Article Khan Academy
 As Hemoglobin Loads Oxygen Its Affinity For H Declines Hydrogen Ions Dissociate From Hemoglob Human Anatomy And Physiology Medical Knowledge Medical Studies
As Hemoglobin Loads Oxygen Its Affinity For H Declines Hydrogen Ions Dissociate From Hemoglob Human Anatomy And Physiology Medical Knowledge Medical Studies
 The Blood Buffer System Youtube
The Blood Buffer System Youtube



