Define Buffer Capacity
Buffer capacity is the amount of acid that buffers are capable of absorbing prior to breaking the capacity for adding strong acid. The pH of intracellular fluids 60 69 is nearer to the PK a of the phosphate buffer.
If you remember high school chemistry or took a college course like Chemistry 101 you will have conducted a titration test.
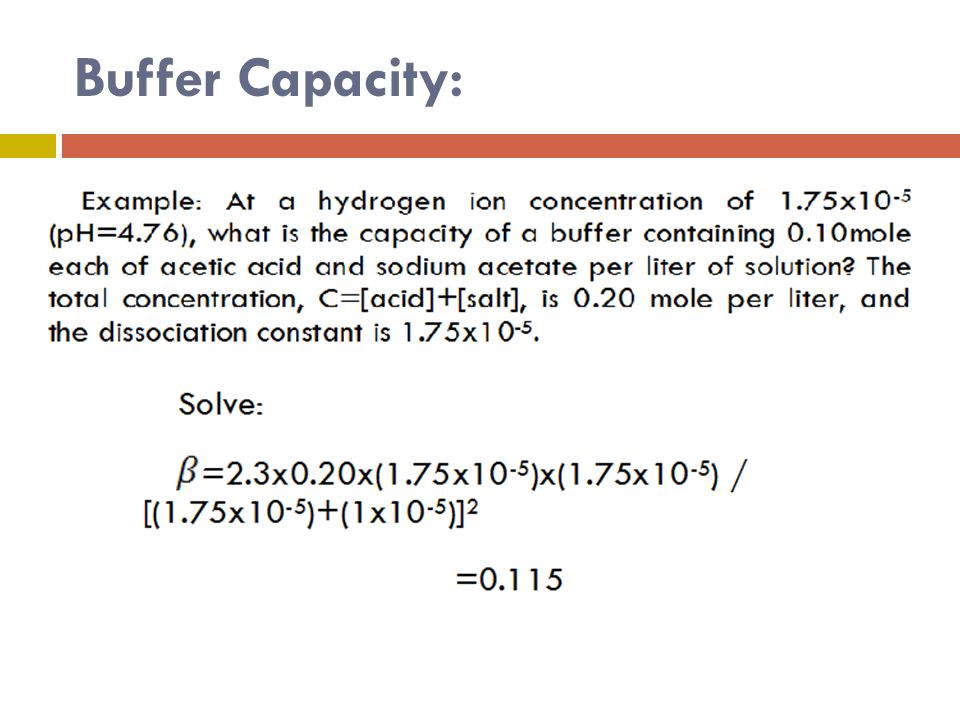
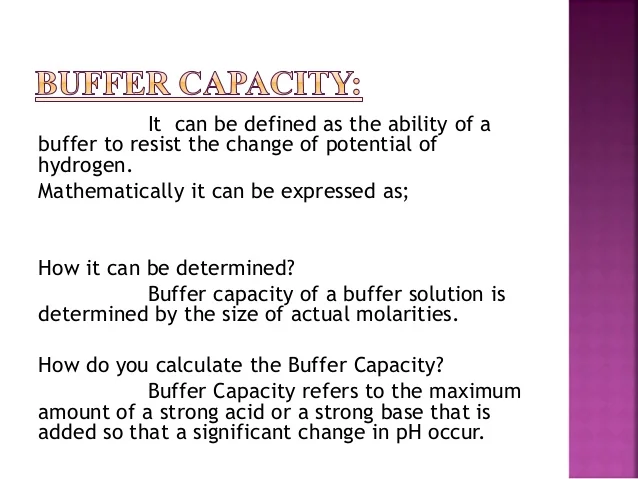
Define buffer capacity. Such definition - although have its practical applications - gives different values of buffer capacity for acid addition and for base addition unless buffer is equimolar and its pHpK a. Buffer Capacity Buffers are characterized by the pH range over which they can maintain a more or less constant pH and by their buffer capacity the amount of strong acid or base that can be absorbed before the pH changes significantly. To elaborate if we take acid or base and add it to a buffer system there will be a change in the pH.
Something or someone that helps protect from harm. Mathematical Define buffering capacity. The buffering region is about 1 pH unit on either side of the pK a of the conjugate acid.
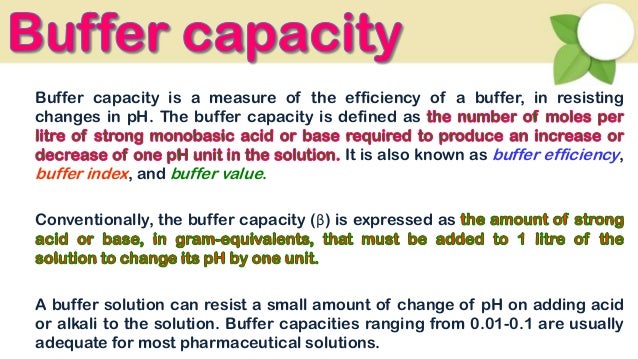
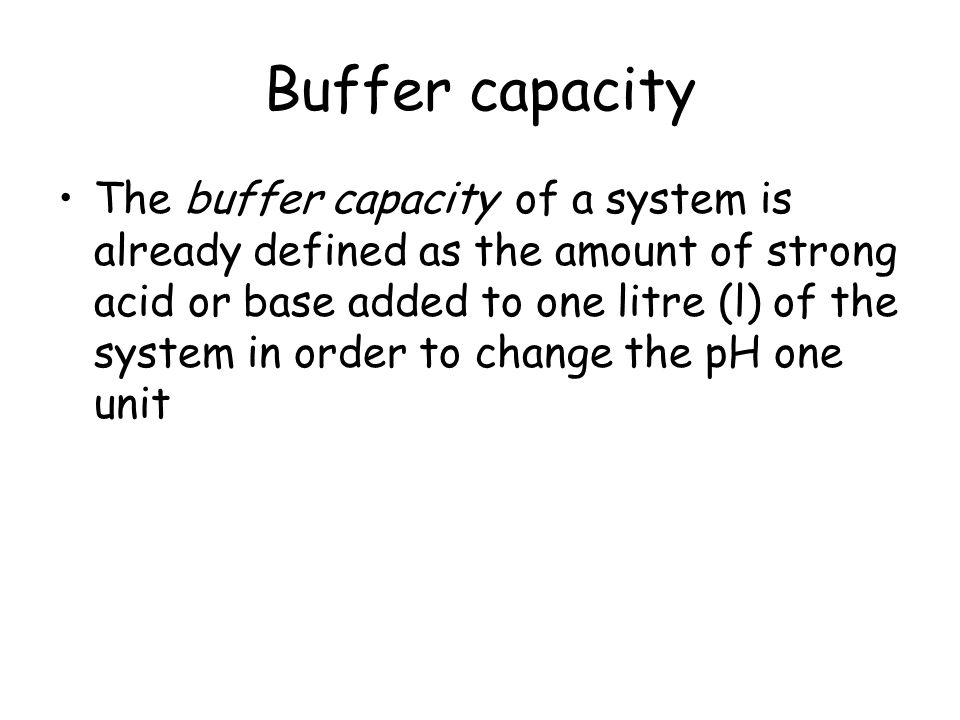
Buffer capacity is a quantitative measure of the resistance to change of pH of a solution by camerino italy containing a buffering agent with respect to a change of acid or alkali concentration. The buffer capacity is a quantity in resisting the pH change at the time of addition of an acid or base. The buffer capacity can also be defined as the amount of mole of strong base needed to change the pH of 1 L of solution by 1 pH of unit.
The Buffer Capacity is a measure of resistant a particular solution is resistant to change in pH when an acid or a base is added to it. 10 MNa HPO and 10 M NaHiPO. An example of a buffer solution is bicarbonate in blood which maintains the bodys internal pH.
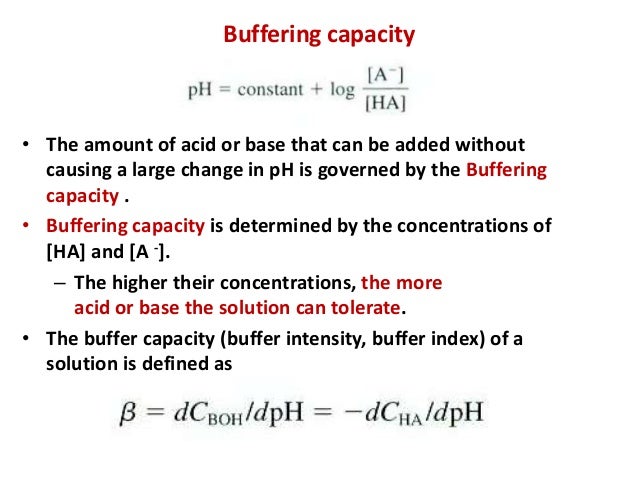
The solution either absorbs or removes H and OH- ions. These supplies often include the raw materials needed for production and also the inventories of finished products waiting for shipment. For a buffer solution an acid with a pKa value close to the buffer solution itself should be chosen which ensures that each part of the conjugate base-acid pair is close to equal.
The higher the acid concentration of the buffer then the buffer capacity will be higher as well. How to use buffer in a sentence. Buffering capacity is defined as the number of moles of strong base or acid needed to change the pH of a liter of buffer solution by one unit.
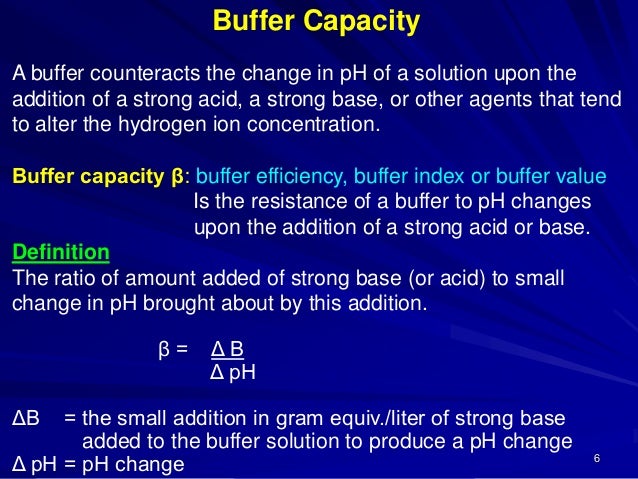
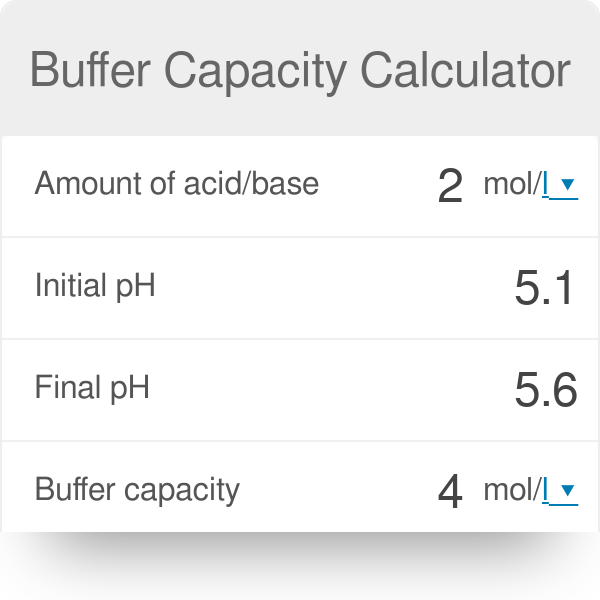
A general buffer capacity estimate is 40 percent of the total sum of the molarites of the conjugate base and acid. Buffer capacity β is defined as the moles of an acid or base necessary to change the pH of a solution by 1 divided by the pH change and the volume of buffer in liters. Buffer capacity can be also defined as quantity of strong acid or base that must be added to change the pH of one liter of solution by one pH unit.
Buffer capacity is the amount of acid or base that can be added before the pH of a buffer changes. It can be defined as follows. Therefore the buffering capacity of the phosphate buffer is highly elevated inside the cells and the phosphate buffer is also effective in the urine inside the renal distal tubules and collecting ducts.
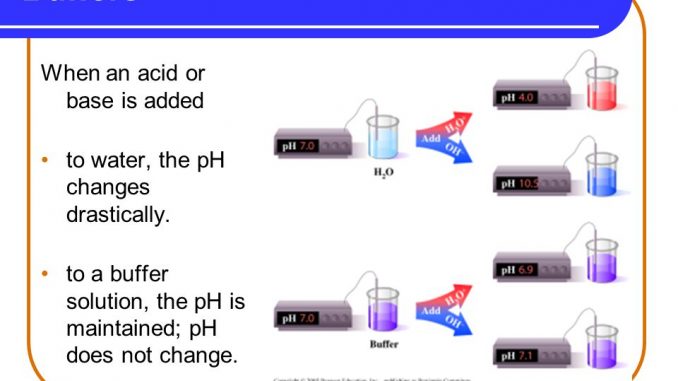
A buffer resists changes in pH due to the addition of an acid or base though consumption of the buffer. Solutions with higher amounts of weak acid have higher levels of buffer capacity when adding a strong base. Solutions with a weaker base have more buffer capacity when adding a strong acid.
In manufacturing the concept of buffering is defined as maintaining enough supplies to keep operations running smoothly. A titration curve visually demonstrates buffer capacity where the middle part of the curve is flat because the addition of base or acid does not affect the pH of the solution drastically. In streaming audio or video from the Internet buffering refers to downloading a certain amount of data before starting to play the music or movie.
Buffer capacity A measure of the resistance of a buffer solution to pH change upon addition or removal of hydroxide ions. How do they differ in pH. Buffer capacity can be defined as the ability of a solution to resist rapid changes in pH.
Having an advance supply of audio samples or. 001 MNazHPO and 001 MNaHzPO4 Buffer b 010 MNa HPO d 010 M NaHPO. Buffer definition is - fellow man.
The metal parts at the front and back of. The change can be either large or small. How do the following buf- fers differ in buffering capacity.
It is a unitless number. 2012 Farlex Inc.
 Acid Base Balance And Buffer Systems In The Human Body Ppt Video Online Download
Acid Base Balance And Buffer Systems In The Human Body Ppt Video Online Download
 Buffer Capacity Chemistry Definition And Formula Az Chemistry
Buffer Capacity Chemistry Definition And Formula Az Chemistry
What Is The Formula For Buffer Capacity Quora
 What Is Buffer Capacity Youtube
What Is Buffer Capacity Youtube
 How To Calculate Buffer Capacity
How To Calculate Buffer Capacity
 Buffer Capacity Definition Examples Diagrams
Buffer Capacity Definition Examples Diagrams
 Buffer Buffering Capacity Properties Of Good Buffer And Role Of Buffer In Vitro And In Vivo Online Biology Notes
Buffer Buffering Capacity Properties Of Good Buffer And Role Of Buffer In Vitro And In Vivo Online Biology Notes
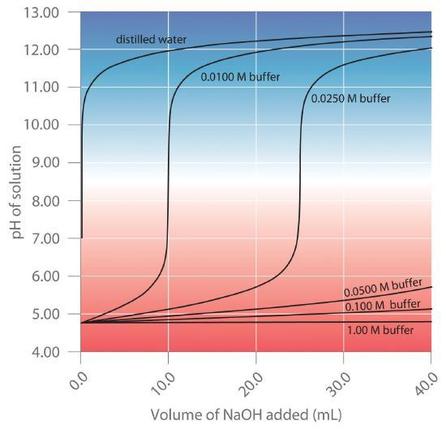 8 9 Buffer Capacity And Buffer Range Chemistry Libretexts
8 9 Buffer Capacity And Buffer Range Chemistry Libretexts
 Buffer Capacity Video Buffer Solutions Khan Academy
Buffer Capacity Video Buffer Solutions Khan Academy
 Exploring Buffers And Buffer Capacity Intro Theory Youtube
Exploring Buffers And Buffer Capacity Intro Theory Youtube
 Buffer And Isotonic Solution Ppt Video Online Download
Buffer And Isotonic Solution Ppt Video Online Download
 What Is Buffer Capacity And What Is The Condition For Maximum Buffer Capacity Youtube
What Is Buffer Capacity And What Is The Condition For Maximum Buffer Capacity Youtube
 Introduction To Buffers Chemistry Libretexts
Introduction To Buffers Chemistry Libretexts